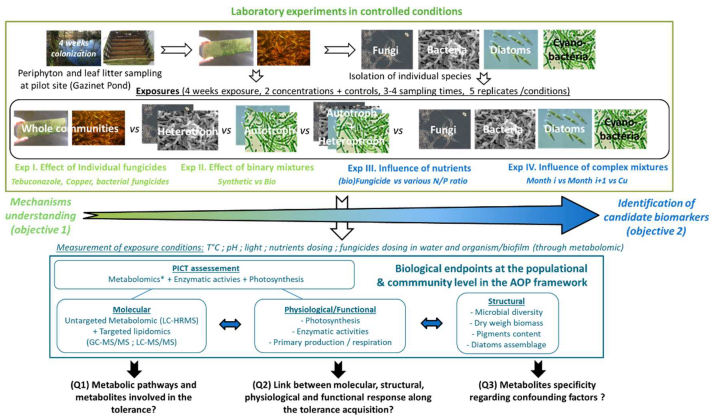
Methodology
MEMENTO aims to decipher the tolerance of aquatic biofilms to bio vs synthetic fungicides. To this end, laboratory experiments in controlled conditions will be conducted to identify adaptive mechanisms of auto-heterotrophic microbial assemblages to fungicides. The responses and tolerance of biofilms will be assessed through the implementation of HRMS-based untargeted metabolomics approach combined with more conventional physiological, structural and functional descriptors and PICT measurement as shown in Figure.
In its concept MEMENTO methodology is organized along an increasing degree of complexity in terms of experiments (from testing single chemical effects to the investigation of confounding factors) and in terms of endpoints and benthic substrata (from the molecular to the physiological/functional and structural responses, from the population to the community responses). Altogether, by unravelling the metabolic pathway and metabolites involved in the tolerance (Q1), the link between molecular/physiological/structural/functional responses (Q2), and the influence of environmental factors on its tolerance (Q3), these investigations will decipher mechanisms of tolerance (O1) acquisition and identify molecular descriptors as candidate biomarkers (O2). Unraveling tolerance acquisition The first and second experiments (Exp I-II) will consist in the exposure to individual and binary mixtures of (bio)fungicides, respectively. First periphyton and leaf litter microbial communities will be exposed to individual (bio)fungicides in order to determine tolerance acquisition (Exp I). Then, fungicides for which PICT is observed will be further tested (alone and as binary mixtures) on the whole communities vs their individual components (fungi, bacteria, diatom, and cyanobacteria) vs co-culture of individual components (fungi + bacteria or diatom + cyanobacteria) (Exp II). In case of PICT detection, the comparison of the metabolomic responses between the control and the exposed biofilm using chemometric methods will allow to identify the metabolites as well as the signalling pathways involved in this acquisition of tolerance (Q1) and to link them to structural and functional responses of the community (Q2). In the same way, the comparison of metabolome/lipidome profiles with the other descriptors at the different sampling dates will provide further evidence about the sequences of events involved in tolerance acquisition (Q2). In addition, the comparison of the metabolomic response following exposure to the different fungicides and their mixture will help to identify similar patterns of pathways and associated metabolites involved in the acquisition of tolerance (i.e. tolerance outcome pathways) supporting the identification of candidate biomarkers of tolerance but also highlighting co-tolerance mechanism (i.e. shared mechanisms). Moreover, the comparison of the response between the whole communities vs co-culture vs monoculture will provide better understanding of individual components acclimation and the role of microbial interactions in this process of tolerance acquisition (Q2). Altogether, these results will provide evidence about the lower/higher/similar ecotoxicity of biofungicides in comparison to synthetic ones. Based on the PICT results, periphytic or leaf-associated microbial communities will be selected for further investigations in Exp III and IV Influence of environmental factors on tolerance acquisition The third experiment and fourth experiments will evaluate the influence of environmental factors on the acquisition of tolerance by microbial communities to biofungicides. First, the influence of nutrients availability will be evaluated on the selected microbial community (periphyton or leaf litter) vs its associated mono/co-culture (Exp III). To do so, the biofilm and its components will be exposed to one model biofungicide with different water N/P ratios according seasonal nutrient concentration variations associated to the use of fertilizers in agriculture areas. Then, because aquatic microbial communities are also exposed to pesticides mixtures inputs resulting from successive applications in agriculture soils, we will further evaluate the influence of a first environmental complex mixture (collected in month m) on the tolerance to (i) a second mixture (collected in month m+1) and (ii) model fungicide (Exp IV). Overall, the comparison of metabolomic profiles will help to identify various tolerance outcome pathways depending on the environmental conditions tested (Q1). This will also highlight co-tolerance phenomena and potential similar tolerance outcome pathways. This will further provide evidence about the specificity of metabolites and so their relevance as candidate biomarkers (Q3), in particular through the parallel investigation of structural/physiological/functional descriptors in order to confirm their link with the molecular response under various conditions tested (Q2). Also, the characterization of the response of the individual components as mono- and co-culture will permit to identify specific pathways and to unravel their contribution to the global response at the community level (Q1, Q2). Moreover, these experiments will help to characterize the relevance of the identified signalling pathways and candidates biomarkers regarding tolerance acquisition following exposure to chemical mixtures (Q1, Q2 and Q3). Biological models and endpoints in the AOP framework MEMENTO will use a combination of classic descriptors and innovative ones following the AOP concept in order to unravel the link between the molecular/biochemical responses, the physiology/function and the structure the biofilms toward highlighting ecosystem tolerance outcome pathways to bio vs synthetic fungicides. Also, the selected endpoints will englobe each major component of the biofilms at different levels. Indeed, while the untargeted metabolomics approach will provide a comprehensive view of the physiological status of the whole biofilm, the physiology/functional responses will encompass both autotrophic (photosynthetic efficiency, primary production) and heterotrophic component (enzymatic activities, respiration). In particular, enzymatic activities (β-glucosidase, laccase, leucine-aminopeptidase, alkaline phosphatase) will allow to assess dissolved organic matter decomposition, including cellulose, lignin, peptides and organic phosphorus compounds, performed by microbial heterotrophs, respectively. Also, the response at the structural level (i.e. both taxonomic composition through metabarcoding and chemical composition through lipidomics) will be assessed through the characterization of the microbial diversity of prokaryotes and eukaryotes (metabarcoding 16S and 18S, nuclear ribosomal in-ternal transcribed spacer, mitochondrial Cytochrome Oxidase I). The overall structure of the whole biofilm will be investigated as well through the characterization of the biomass (Dry weight and Ash Free Dry weight), population dynamics (cell densities, mortality) and the use of targeted lipidomics (structural vs energetic lipids composition). Microscopic observation will be also performed on the whole biofilm to get the taxonomic composition of specific assemblage (e.g. diatoms). Other structural descriptors will include bacterial density per flow cytometry, fungal biomass (i.e. ergosterol dosing) and algal biomass (i.e. chl-a dosing). In addition to the biofilms, some of these descriptors (untargeted metabolomics, targeted lipidomics, photosynthesis, enzymatic activity, tolerance) will be also investigated on monospecific and co-cultures cultures to unravel their contribution to the whole response of the biofilm.